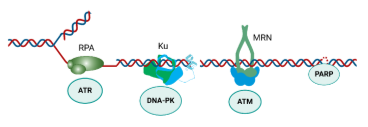
The DNA Damage Response (DDR) is an integrated network of DNA repair and cell signaling pathways that are critical for maintaining genomic stability. The success of PARP inhibitors has spurred the development of numerous DDR-focused therapeutic programs, the majority of which target kinases. However, the clinical outcomes of these approaches have not met expectations. We have pursued the novel approach of intervening upstream of the DDR kinases, targeting specific DDR sensors by developing agents to block protein-DNA interactions as a therapeutic strategy.
The human ssDNA binding protein, replication protein A (RPA), is a critical sensor of ssDNA that activates the DDR in response to replication stress. We have discovered and developed a novel small molecule RPA inhibitor (RPAi) NERx-329 that blocks the RPA-DNA interaction and elicits a state of chemical RPA exhaustion, resulting in in vivo monotherapy anticancer activity. Genetic analyses reveal a series of alterations in DNA repair, recombination, and replication that result in increased sensitivity to RPA inhibition providing insight into the DDR and the potential to target unique patient populations.
Ku is a sensor of DSBs that binds to DNA ends and activates DNA-PK. Inhibiting DNA-PK is a common strategy to block DSB repair, enhancing the efficacy of therapies like ionizing radiation (IR). We have developed Ku-DNA binding inhibitors (Ku-DBis), which inhibit DSB repair and DNA-PK autophosphorylation resulting in increased sensitivity to IR. We have discovered a novel series of Ku-DBis with improved cellular uptake and bioavailability. These inhibitors show variable anticancer effects across a series of non-small cell lung cancer (NSCLC) cell lines, with ATM-null cells showing the most sensitivity. The efficacy of Ku-DBis assessed alone and in combination with IR in a human xenograft model of NSCLC demonstrates that Ku-DBi treatment blocks IR-dependent DNA-PKcs autophosphorylation, modulates the DDR, and reduces tumor cell proliferation. This work is the first in vivo assessment of an optimized novel Ku-DBi and these data represent a significant advance in the development of Ku-DNA binding inhibitors and their potential therapeutic utility for cancer treatment.