Affiliation: Department of Pharmaceutical Sciences and Experimental Therapeutics, University of Iowa College of Pharmacy
Abstract: Some of the most potent chemotherapeutic drugs cannot be used as-is due to their unacceptable off-target side effects. One common solution to this issue is to covalently link the drug molecule to biomolecules or biodegradable polymers to allow targeted drug delivery to solid tumors, thus reducing the off-target toxicities and enabling clinical use.
In this study, we synthesized a polymer-drug conjugate (PDC) comprising of poly(lactic-co-glycolic acid) (PLGA), a biodegradable polymer, and monomethyl auristatin E (MMAE), a highly toxic drug. The MMAE content in the PDC was analyzed using ¹H-NMR and PLGA-calibrated GPC techniques. The PDC was used in combination with PLGA derivatives to fabricate nanoparticles. The nanoparticle surface was subsequently decorated with cetuximab, giving tumor-targeted nanoparticles (TTNPs). Cetuximab is a clinically relevant monoclonal antibody that targets human epidermal growth factor receptor (EGFR) with an indication for colorectal cancer treatment. Similar nanoparticle fabrication was also performed without cetuximab to make non-targeted nanoparticles (NTNPs).
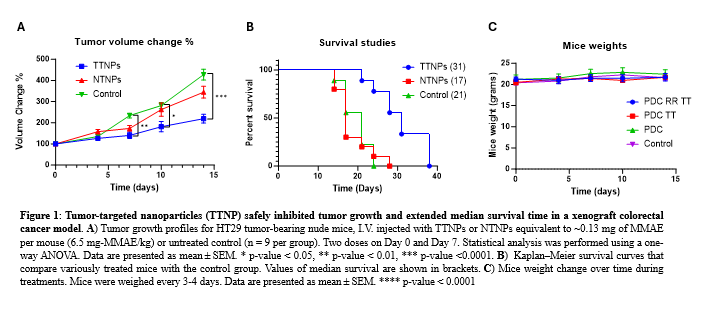
The anti-tumor efficacy of NTNPs was assessed in vitro in a HT-29 human colorectal cancer cell line and in vivo in a heterotopic HT-29 xenograft mice model. The tumor-bearing mice (~200 mm³ tumor volumes) were treated twice weekly for two consecutive weeks with either TTNP, NTNP, or left untreated via intravenous injection. The tumor volume was measured throughout the treatment, and the survival rate of the animals was tracked after treatment cessation. The animal body weight was also monitored for safety.
We found that the mice treated with TTNP showed significant tumor growth inhibition compared to the NTNP and the untreated control. TTNP treatment also resulted in an extended median survival time (31 days) compared to the NTNP group (17 days) and untreated controls (21 days), with no appreciable weight loss. These findings support the potential of TTNP for further development as a safe and efficacious treatment for colorectal cancer.
Authors: Pornpoj Phruttiwanichakun1, Mohammad A. Al-Natour1, Ramkrishna Sen1, Sean M. Geary1, Aliasger K. Salem1