Affiliation: Department of Pharmaceutical Sciences & Experimental Therapeutics, College of Pharmacy, University of Iowa
Abstract: Despite the increasing availability of protein products including monoclonal antibody (mAb) drugs and their proven success in treatment of rheumatoid arthritis, multiple sclerosis, asthma, atopic dermatitis, and many other diseases, the early identification of the protein candidate molecules with desirable manufacturability, stability and delivery attributes remains a big challenge. Self-association and poor solution behavior, manifested in high viscosity/opalescence at relevant concentrations or in phase separation and stability issues, are major limiting factors in development of protein products.
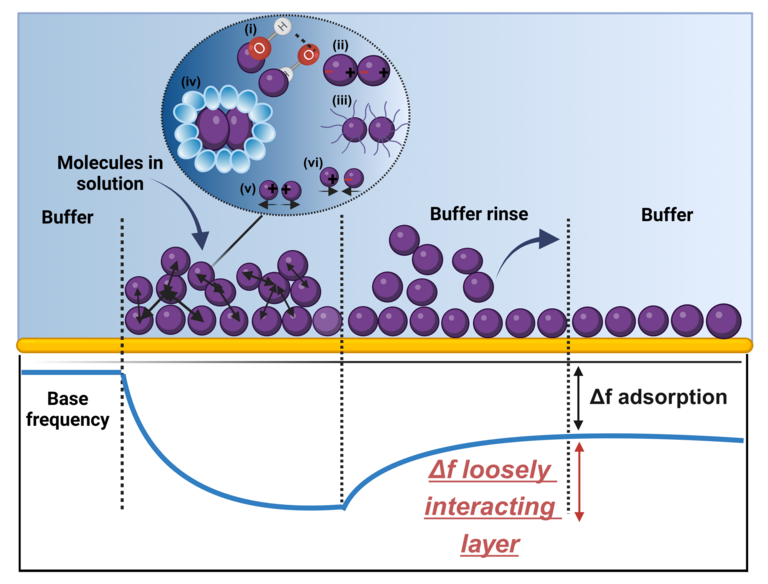
Solution behavior is believed to be governed by protein self-association, however, measurement of these associations experimentally and prediction of the solution behavior are challenging using the current methods. The quartz crystal microbalance with dissipation monitoring (QCM-D) technique often detects a loosely interacting layer on top of the irreversibly adsorbed layer of molecules/particles. In this work, we demonstrate that this layer provides a plethora of information about molecular/particulate self-association and can be used to predict colloidal stability of macromolecular systems and solution behavior of monoclonal antibodies and other proteins. Unlike currently used approaches, this method is capable of measuring the metrics of protein self-association under relevant formulation conditions. In addition, we show that the reliability of this method is significantly superior to that of DLS-based diffusion interaction parameter.
Authors: Yusra Rahman, Siddhanth Hejmady, and Reza Nejadnik (All Authors contributed equally)